ESG Software for Pharmaceutical Companies
Fair Supply empowers pharmaceutical manufacturers, distributors and research organisations to map their global supply chains, quantify ESG risks across complex production networks and lead the sector in transparency, safety and responsible operations.
.avif)
Pharmaceutical and life sciences companies around the world trust Fair Supply to strengthen supply chain governance and ESG performance.


Turn ESG obligations into a strategic advantage
Rather than treating compliance as a checkbox exercise, pharmaceutical leaders are using ESG insights to manage risk across highly regulated, globally distributed manufacturing and sourcing networks, strengthening supply continuity, regulatory confidence and long-term operational resilience.
Stay ahead of regulation
Keep pace with rapidly evolving ESG frameworks, from ISSB and climate reporting to global modern slavery legislation, and remain audit ready across jurisdictions where you manufacture, distribute and source raw materials.
Build investor and stakeholder trust
Demonstrate your commitment to ethical pharmaceutical production with credible, data-driven insights that withstand scrutiny from boards, regulators, customers and healthcare partners.
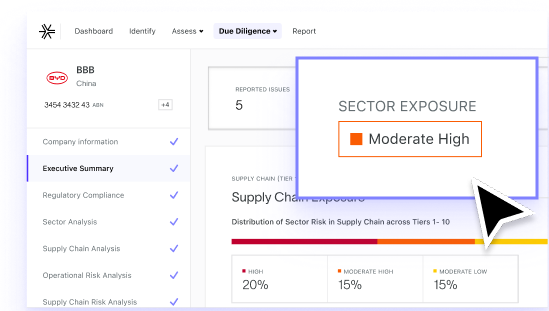
Uncover hidden risks in your supply chain
Pinpoint suppliers and outsourced manufacturers who are most exposed to ESG risks before they become liabilities across Active Pharmaceutical Ingredients (API), excipients, packaging, laboratory equipment, contract manufacturing, logistics and cold chain distribution services.
Manage multiple risk types, all in one platform
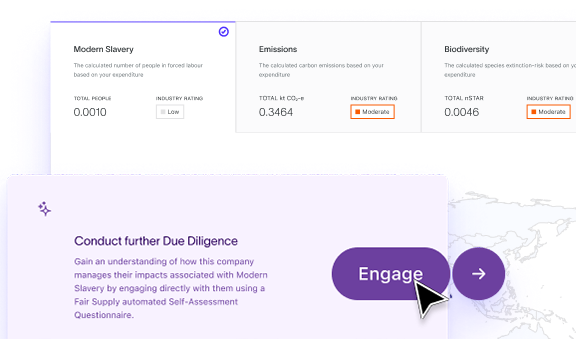
Modern Slavery
Understand human rights risks and stay aligned with current and emerging regulations in any region.
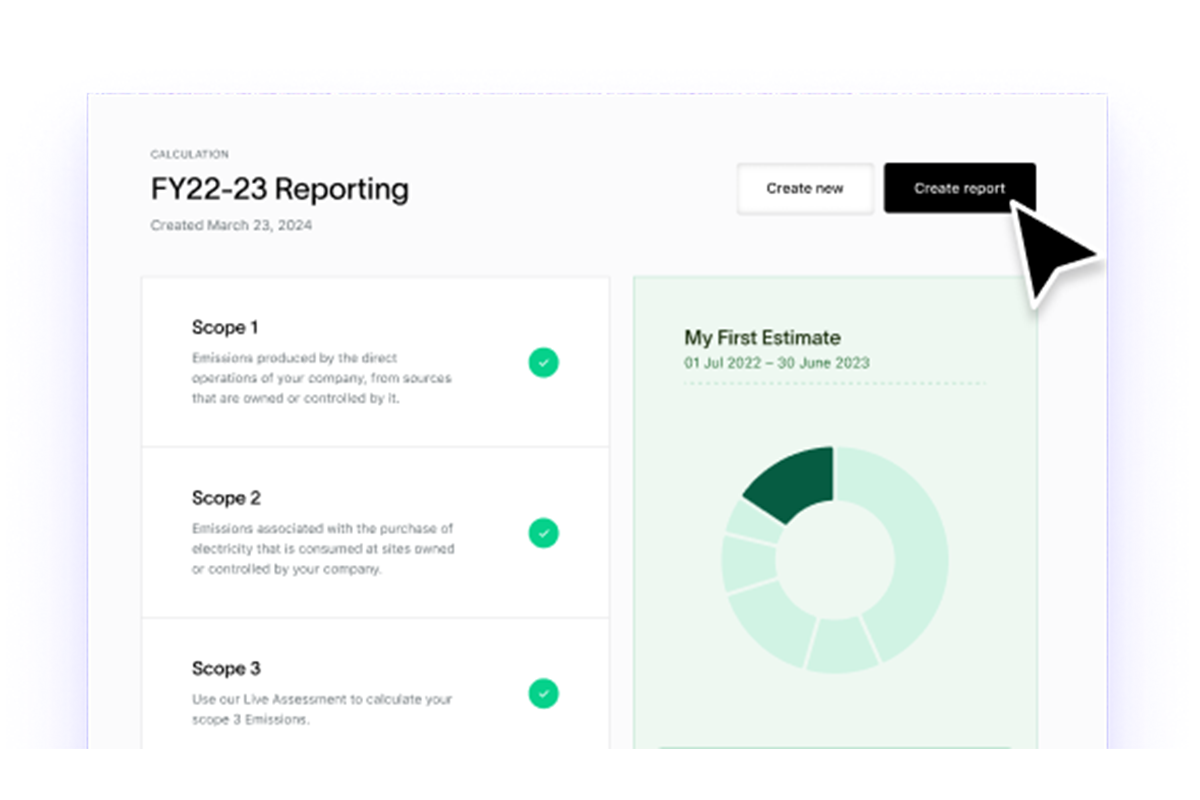
Carbon Emissions
Model Scope 1, 2 and 3 emissions across your supply chain and produce fast disclosures and regulator-ready reports.
Biodiversity Impact
Measure environmental destruction throughout your supply chain and proactively reduce your impact to stay ahead of evolving regulations and customer concerns.
Global Trade Risk
Use our tariff calculator to understand the impact of global trade policy shifts on your supply chain costs.
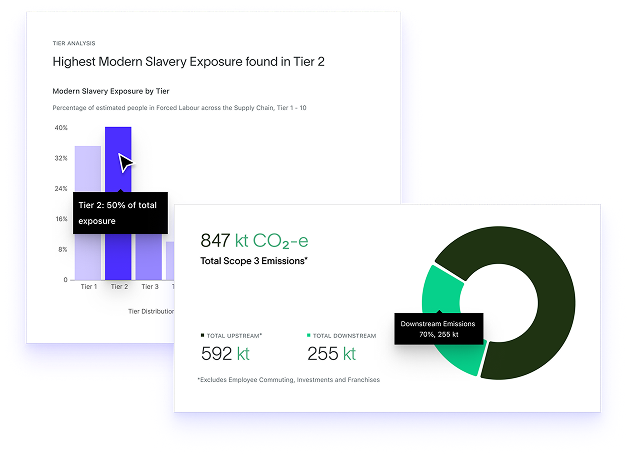
Global Supply Chain Mapping
Visualise every tier of your pharmaceutical supply network with high resolution data designed to uncover ESG risk in complex, globalised production systems.
Fair Supply uses proprietary modelling to trace exposure beyond direct suppliers and reveal indirect and deep tier relationships that are common in API production, chemical processing, packaging and distribution.
Engage Suppliers at Scale
Centralise how you assess, engage, and document supplier ESG performance. Standardise SAQs, track responses, and maintain a clear, auditable record of activity.


FAQs
Why do pharmaceutical companies need ESG software?
Pharmaceutical supply chains are highly globalised and exposed to ESG risks including modern slavery in raw material extraction, emissions from chemical processing, biodiversity impacts and complex outsourced manufacturing models. ESG software provides a defensible and systematic way to quantify these risks across suppliers and jurisdictions, supporting regulatory obligations and internal governance.
How does ESG software improve real world usability for procurement and supply teams?
Pharmaceutical procurement teams manage large supplier ecosystems with tight compliance demands. Fair Supply requires only supplier name and spend to generate multi-tier ESG risk insights. Dashboards convert complex modelling outputs into clear, operationally useful information suitable for supplier risk reviews, GMP- related governance processes and regulatory reporting.
How does Fair Supply support Tier 10 visibility for pharmaceutical supply chains?
Pharmaceutical supply chains involve many layers such as chemical producers, API manufacturers, contract manufacturing organisations, packaging suppliers and logistics providers. Fair Supply maps risk up to 10 tiers to expose upstream vulnerabilities that supplier self reporting cannot reveal. This helps ESG, procurement and compliance teams identify risks embedded deep within global production systems.
How does Fair Supply support ESG reporting and compliance for pharmaceutical companies?
Pharmaceutical companies face strict reporting obligations including CSRD, CSDDD, TCFD, ISSB and modern slavery laws. Fair Supply produces audit- ready ESG reports that draw on supplier risk assessments to create a single source of truth for disclosures to regulators, investors, auditors and internal committees.
How does Fair Supply support Scope 3 emissions screening for pharmaceutical supply chains?
Scope 3 emissions in pharmaceuticals arise from chemical manufacturing, energy intensive processing, packaging, logistics, refrigeration, laboratory operations and outsourced manufacturing. Fair Supply automates Scope 3 screening through spend classification, defensible emissions factors and reporting- ready summaries. This enables rapid identification of emissions hotspots to support decarbonisation initiatives.
How does ESG software enhance supplier due diligence in the pharmaceutical sector?
Pharmaceutical companies rely heavily on outsourced manufacturing, contract research organisations and specialised suppliers. Fair Supply standardises due diligence through configurable SAQs that assess modern slavery, emissions and biodiversity. Responses feed into risk scoring and create an auditable record suitable for internal audits, GMP- related supplier reviews and regulatory compliance.
How does Fair Supply help pharmaceutical organisations assess biodiversity related risks?
Pharmaceutical supply chains are connected to biodiversity impacts through chemical feedstocks, agricultural inputs, packaging materials and global logistics operations. Fair Supply provides supplier specific biodiversity risk scoring using the nSTAR methodology and connects to datasets such as the IUCN Red List and IBAT. This supports TNFD, ISSB and CSRD aligned reporting.
What makes Fair Supply data defensible for audits and regulatory reviews in pharmaceuticals?
Pharmaceutical companies require highly defensible and transparent ESG data due to regulatory oversight and global compliance obligations. Fair Supply uses independently assured methodologies, globally validated datasets and clear audit trails. Every insight aligns with major ESG frameworks and supports regulatory submissions, GMP compliance reviews and board reporting.